Enzymatic Structure
The secondary structure of a single subunit contains a 9 beta sheet parallel backbone wrapped by 9 large alpha helices. Near the sodium bound end, 4 small anti-parallel beta sheets and 1 small alpha helix enable a turn in the residue chain(small turn). Opposite the sodium bound ligand, 6 alpha helices point towards a common point, three on each side of the beta sheet backbone. The alpha helices form a small groove for a NAD+ cofactor to attach near the beta sheeting. The structure most nearly resembles an alternating alpha/beta classification. As for the 3D structure, the enzyme forms a sort of crevice for the substrate to bind. (taken from proteopedia Jan 2017)
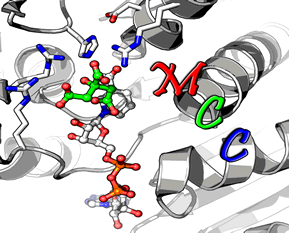
Mechanism and Active site
Select mdh publications and links
- Worthington Biochemistry MDH page
- MDH PDB Collection
- Malate Dehydrogenase: A Model for Structure, Evolution and Catalysis
- Molecular Evolution Within the L-Malate and L-Lactate Dehydrogenase Super-Family
- Plant Rood MDH
- Structural Analysis of MDH with a Variable Active Site
- Glyoxosomal MDH
- Substrate Inhibition of MDH
- The Effects of Adenine Nucleotides on Pig Heart Malate Dehydrogenase
- Apple MDH cold tolerance and salt stress
- MDH and trichomonads
- MDH and LDH in Trichomona vaginalis
- MDH and Aconitase/Citrate Synthase Interaction
- Channeling of MDH and CS
- Interaction between CS and MDH
- Sigma Aldrich Enzyme Background