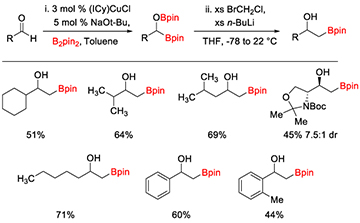
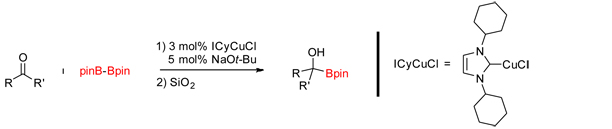
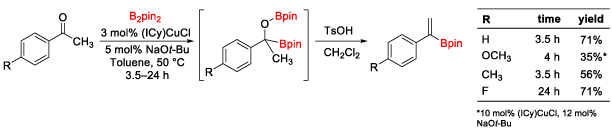
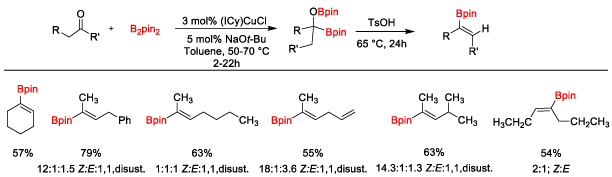
research description The Clark research group focuses on the applications of organometallic chemistry to organic synthesis. Organometallic complexes are used as catalysts to mediate organic reactions that either cannot be achieved without a metal, or are much more efficient and practical with the metal present. The goals of the research projects are to develop new catalysts that are synthetically useful and ultimately to apply the newly developed reaction to a synthetic target. Projects in my group range from synthetic organometallic chemistry (design of new transition metal catalysts) to new organic reaction development using transition metal catalysts. The focus of our research is on the introduction of boron substituents into organic molecules. New methods to incorporate boron into organic molecules are valuable synthetic tools to develop because the C–B bond is so versatile and can be converted into C–C, C–O, C–N, and other C–X bonds. Therefore, new methods to introduce boron into organic molecules will simplify the synthesis of complex targets such as pharmaceutical drugs, natural products, organic materials, and organocatalysts. Pharmaceuticals containing boron are also recieving significant attention in recent years, leading to several FDA-approved drugs for the treatment of cancer and bacterial fungal infections, among other applications. Undergraduate Student-Focused Research Program The University of San Diego is a Predominantly Undergraduate Institution (PUI) and the Department of Chemistry and Biochemistry does not have graduate students. The majority of the work done in the Clark research group is by undergraduate students, providing valuable hands-on training in synthetic organic and organometallic chemistry. This experience provides students with added confidence and ability to succeed in a career in chemistry or biochemistry or in graduate school. Students take ownership of their research project and are slowly guided toward independence. Professor Clark has mentored over 70 undergraduate students, 12 high school students, and 6 post-doctoral research associates since 2007. Students have gone to and excelled in graduate programs such as UCLA, UC Irvine, Colorado State University, UNC Chapel Hill, Scripps Research Institute, University of Michigan, UCSC, UCSB, Rutgers University, Oregon State University, and University of Delaware. Many students have also gone straight into the biotechnology workforce, medical school, and other career paths that utilize their science background. Dr. Clark welcomes applications from undergraduate students from within and outside of USD. Please contact him by email (clarkt@sandiego.edu). Professor Clark also mentors postdoctoral research associates that are interested in a career at a PUI when funding is available. Please contact Professor Clark for additional information on opportunities in his research group. Copper-Catalyzed Diboration or Carbonyl Compounds Recently, the applications of alpha-heteroatom-substituted boronate esters in asymmetric synthesis and as pharmaceutical targets have necessitated a general way to form these intermediates. Our group is interested in using copper-catalyzed diboration reactions to generate alpha-hydroxy-substituted boronate esters (Scheme 1). Thus far, we have developed improved conditions for the diboration of aldehydes and ketones based on the copper catalyst originally reported by Sadighi and co-workers, providing the first example of tertiary alpha-hydroxyboronate esters.1 We have also made progress in developing new methods to utilize the alpha-hydroxyboronate esters as synthetic intermediates. Scheme 2. Acid-Catalyzed Elimination of Alpha-Hydroxyboronate Esters The synthetic utility of ketone diboration products was demonstrated in the acid-catalyzed elimination to generate 1,1-disubstituted (Scheme 2) and trisubstituted vinylboronate esters (Scheme 3) with good control of the olefin geometry in most cases. The reaction conditions are simple and easily reproduced through a two-step, one-flask reaction sequence. The vinylboronate esters have been subjected to Suzuki-Miyaura coupling conditions with aryl halides to provide a variety of styrene derivatives (not shown).2 Scheme 3. Synthesis of Trisubstituted Vinylboronate Esters We have also utilized aldehyde diboration products in the Matteson homologation reaction. Initially it was thought that the alpha-hydroxy substituent would require protection prior to homologation, but direct homologation with excess LiCH2Cl was developed, providing beta-hydroxyboronate esters in good to high yields in two steps from the aldehydes (Scheme 4).3 We are currently working to expand this homologation reaction to more complex homologating agents. We recently reported the use of allyl chloride as a homologating agent, for example.4 This project has been funded by the Research Corporation for Science Advancement and the National Institutes of Health. Additional directions of this chemistry involve gem-diboronates through a collaboration with Pfizer. Scheme 4. Diboration/Homologation Sequence with Aldehydes
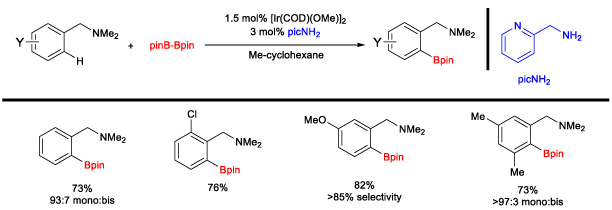
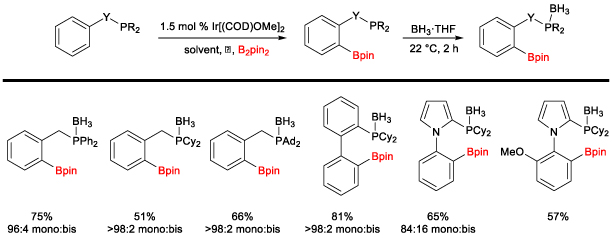
Iridium-Catalyzed C-H Borylation Reactions The second major project in our research group is on substrate-directed C–H borylation reactions using iridium catalysts. The original focus was on the development of a bifunctional ligand that could direct the C–H borylation reaction, which ultimately resulted in a ligand (picolylamine) that induces directed C–H borylation by partial dissociation of one arm of a diamine ligand (Scheme 5).5 Scheme 5. Amine-Directed Ortho C-H Borylation Reaction
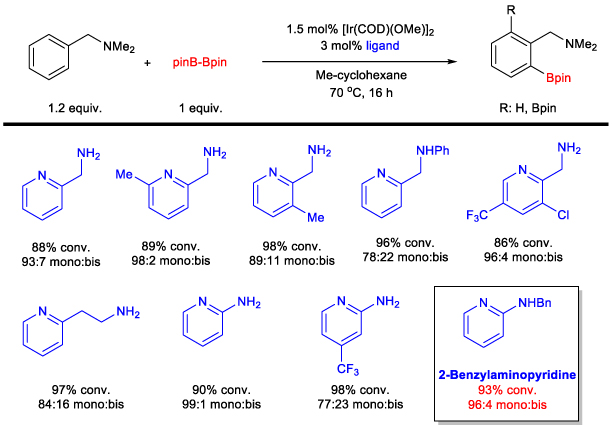
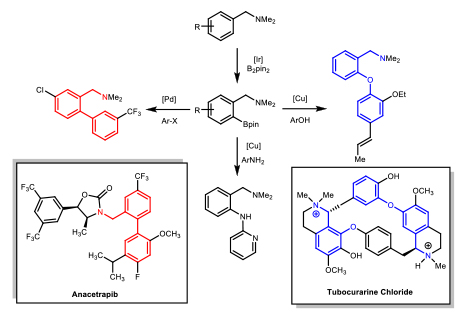
The catalyst efficiency and selectivity were improved (for monoborylation over bisborylation) through a comprehensive study of bidentate ligands with an emphasis on diamino ligands. The role of each nitrogen was explored to probe the partial dissociation of one arm of the ligand. Local steric effects were also examined, demonstrating a significant influence on borylation selectivity. Ultimately, it was found that the diamine bite angle played a significant role in both selectivity and reactivity. A sub-set of ligands is summarized in Scheme 6, demonstrating the steric, electronic, and bite angle effects on conversion and selectivity. Scheme 6. Effect of Ligands on C-H Borylation Selectivity and Conversion 2-(N-Benylamino)pyridine was found to have optimal reactivity and selectivity.6 These studies were central to our initial understanding of the reaction mechanism. We have recently disclosed a full mechonistic study in collaboration with Hairony Guan (University of Cincinnati) and Edwin Webster (Mississippi State University)7. This study revealed the important role of HBion as an auto catalyst in the reaction. Scheme 7. Synthetic Utility of Amine-Directed C-H Borylation
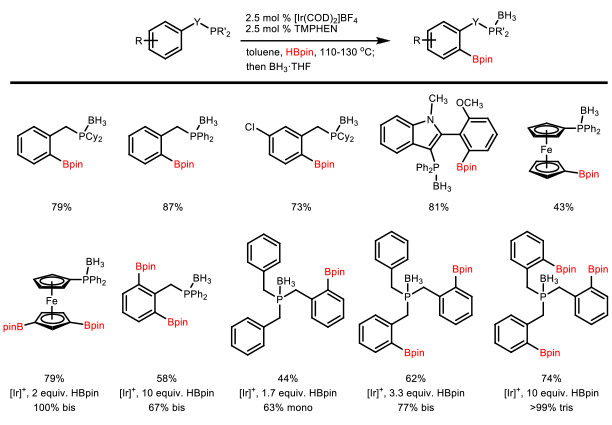
C-H Borylation of Organophosphorus Reagents A recent direction in our group is focused on phosphine-directed C-H borylation reactions which emerged from our studies of the amine-directed C–H borylation reaction described above. The results obtained with the ligand screen (Scheme 6) were used to begin looking at additional directing groups. It was found that phosphines were capable of directing the C–H borylation of arenes. Interestingly, picolylamine was an ineffective ligand to mediate this transformation, but 2-benzylaminopyridine provided moderate conversion. Optimal yields were obtained, however, without the addition of an external ligand. The reaction conditions have been optimized and the resulting phosphine boronate esters were protected as the borane complex to facilitate isolation of the air-sensitive products (Scheme 8). X-ray crystal structures of several protected phosphines have been obtained to confirm the site of borylation. Deprotection conditions have been established to provide the phosphine boronate esters.11 Scheme 8. Phosphine-Directed C-H Borylation/Borane Protection The scope of the reaction was recently expanded by identifying a cationic iridium complex that could catalyze the phosphine-directed C-H borylation. The new system resulted in a wide variety of phosphines that were readily borylated.12 Several phosphines could also be polyborylated in good yields. Deprotection of the phosphines boronates provides interesting ambiphilic phosphine boronates, potential organocatalysts that are currently being examined. Scheme 9. Phosphine-Directed C-H Borylation with Borylation with Cationic Iridium Complex The phosphine-directed C-H borylation chemistry led us to initiate a collaborative project with Professor Don Watson at the University of Delaware. Through this collaboration, we developed the phosphonate-directed C-H borylation of arenes (Scheme 10).13 The weak coordinating ability of phosphonates and the tendency of P(V) groups to be reduced in the presence of boranes necessitated particular reaction conditions that were realized through High Throughput Experimentation at the University of Delaware. The resulting ortho-borylated arylphosphonates provide a valuable synthetic handle to access a variety of biologically relevant arylphosphonates. Scheme 10. Phosphonate-Directed C-H Borylation of Arenes We are currently working to utilize the resulting arylboronate esters in a copper-catalyzed oxidative homocoupling reaction as an expedient method to access biarylbisphosphonates (Scheme 11), which are precursors to important bisphosphine ligands, such as SEGPHOS. Scheme 11. Oxidative Homocoupling of Borylated Arylphosphonates
An extension of this collaboration with the Watson group has involved the nitro-directed C-H borylation of nitroarene, which provides multi-functional arenes for target-directed synthesis. These projects are funded by an NSF RUI grant.
Carbon-Phosphorus Bond Coupling Reactions
Inspired by challenges accessing the arylphosphonates utilized in Scheme 10, we are developing reactions that utilize arylboronate esters for the direct coupling to dialkylphosphites (Scheme 12). Methods are known that utilize arylboronic acids to couple to dialkyl phosphites and secondary phosphine oxides. We found that the varying degree of dehydration of commercially available arylboronic acids to boroxines rendered the coupling reactions unpredictable. This coupled with the recent influx of methods that incorporate boronate esters into organic molecules makes a method that utilizes the arylboronate esters directly more efficient and reliable. This project is currently funded by an American Chemical Society-Petroleum Research Fund grant. Scheme 12. Oxidative Coupling of Arylboronate Esters with Dialkylphosphites References: (1) McIntosh, M. L.; Moore, C. M.; Clark, T. B. Org. Lett. 2010, 12, 1996. |

 rs
rs