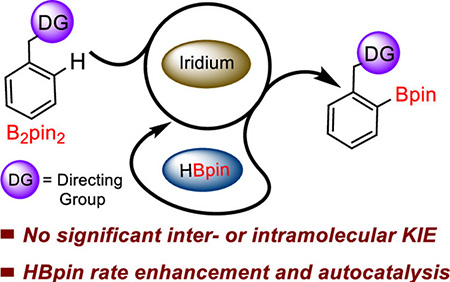
publications Independent Publications 32. Le, N.; *Chuag, N. L.; *Oliver, C. M.; Samoshin, A. V.; *Hemphill, J. T.; *Morris, K. C.; *Hyland, S. N.; Guan, H.; Webster C. E.; Clark, T. B. “Hidden Role of Borane in Directed C-H Borylation: Rate Enhancement through Autocatalysis” ACS Catalysis, 2023, 13, 12877-12893. 31. Fomina, I. A.; Myers, C. R.; Soumis, C. L. M.; Scheuermann, M. L.; McCarty, J.; Clark, T. B.; O’Neil, G. W. “Regiodivergent Medium-Ring Oxasilacycle Synthesis from Diallylsilanes” Heterocycles, 2022, 104, 1966–1993. 30. Myers, C. R.; Spaltenstein, P.; Baker, L. K.; Schwans, C. L.; Clark, T. B.; O’Neil, G. W. “Sequential Iodine-Mediated Diallysilane Rearrangement/Asymmetric Dihydroxylation: Synthesis and Reactions of Enantioenriched Oxasilacycles” Tetrahedron Letters, 2021, 82, 153392. https://doi.org/10.1016/j.tetlet.2021.153392
29. *Auth, M. R.; McGarry, K. A.; Clark, T. B. “Phosphorus-Directed C–H Borylation” Advanced Synthesis and Catalysis, 2021, 363, 2354-2365. https://doi.org/10.1002/adsc.202100173
28. *Morris, K. C.; *Wright, S. E.; Clark, T. B. “Phosphine-Directed sp3 C H, C O, and C N Borylation” Journal of Organic Chemistry, 2020, 85, 14795–14801. https://doi.org/10.1021/acs.joc.0c01706
27. Xu, F.; Duke, O. M.; Rojas, D.; *Eichelberger, H. M.; Kim, R. S.; Clark, T. B.; Watson, D. A. “Arylphosphonate-Directed Ortho C–H Borylation: Rapid Entry into Highly-Substituted Phosphoarenes” Journal of the American Chemical Society, 2020, 142, 11988–11992. https://doi.org/10.1021/jacs.0c04159
26. *Meyer, G. F.; *Nistler, M. A.; Samoshin, A. V.; *Thane, T. A.; *Ferber, C. J.; *McManus, B.; O’Neil, G. W.; Clark, T. B. “ß-Silyloxy Allylboronate Esters through an Aldehyde Borylation/Homologation Sequence” Tetrahedron Letters 2020, 61, 152082. https://doi.org/10.1016/j.tetlet.2020.152082 
25. Clark, T. B.; Cho, H. Y, In Science of Synthesis: Advances in Organoboron Chemistry towards Organic Synthesis, Fernández, E., Ed.; Thieme: Stuttgart, (2019); p 131-182. 24. *Hyland, S. N.; *Meck, E. A.; Tortosa, M.; Clark, T. B. “α-Amidoboronate Esters by Amide-Directed Alkane C–H Borylation” Tetrahedron Letters, 2019, 60, 1096-1098. https://doi.org/10.1016/j.tetlet.2019.03.020 
23. Spaltenstein, P.; Cummins, E. J.; Yokuda, K.-M.; Kowalczyk, T.; Clark, T. B.; O’Neil, G. W. “Chemoselective Carbonyl Allylations with Alkoxyallylsiletanes” Journal of Organic Chemistry, 2019, 84, 4421-4428. https://pubs.acs.org/doi/10.1021/acs.joc.8b03028
22. *Wright, S. E.; *Richardson-Solórzano, S.; Stewart, T. N.; *Miller, C. D.; *Morris, K. C..; Daley, C. J. A.; Clark, T. B. “Accessing Ambiphilic Phosphine Boronates through C-H Borylation by an Unforeseen Cationic Iridium Complex” Angewandte Chemie, International Edition, 2019, 58, 2834-2838. https://doi.org/10.1002/anie.201812857 
21. López, A.; Clark, T. B.; Parra, A.; Tortosa, M. “Copper-Catalyzed Enantioselective Synthesis of β-Boron β-Amino Esters” Organic Letters 2017, 19, 6272–6275. 
https://pubs.acs.org/doi/10.1021/acs.orglett.7b02784 20. Clark, T. B.; Emmerson, D. G.; Honsberger, J. “From the Research Lab to the Classroom: A Multi-Faceted High School Chemistry Outreach Program” from "Educational and Outreach Projects from the Cottrell Scholars Collaborative Professional Development and Outreach Volume 2" ACS Symposium Series, 2017, Vol. 1259, Ch. 6, 69–84. https://pubs.acs.org/doi/10.1021/bk-2017-1259.ch006 19. *Marcum, J. S.; McGarry, K. A.; *Ferber, C. J.; Clark, T. B. “Synthesis of Biaryl Ethers by the Copper-Catalyzed Chan–Evans–Lam Etherification from Benzylic Amines Boronate Esters” J. Org. Chem. 2016, 81, 7963–7969. https://pubs.acs.org/doi/10.1021/acs.joc.6b01254 
18. Clark, T. B. “α-Hydroxyboronate Esters: Formation and Synthetic Applications” Asian J. Org. Chem. 2016, 5, 31–42. https://onlinelibrary.wiley.com/doi/10.1002/ajoc.201500284 
17. McGarry, K. A.; *Duenas, A. A.; Clark, T. B. “Selective Formation of ortho-Aminobenzylamines by the Copper-Catalyzed Amination of Benzylamine Boronate Esters” J. Org. Chem. 2015, 80, 7193–7204. https://pubs.acs.org/doi/10.1021/acs.joc.5b01074 
16. Hale, L. V. A.; Emmerson, D. G.; %Ling, E. F.; Roering, A. J.; *Ringgold, M. A.; Clark, T. B. “An ortho-Directed C–H Borylation/Suzuki Coupling Sequence in the Formation of Biphenylbenzylic Amines” Org. Chem. Frontiers. 2015, 2, 661–664. https://pubs.rsc.org/en/content/articlelanding/2015/qo/c4qo00348a#!divAbstract 
15. Hale, L. V. A.; McGarry, K. A.; *Ringgold, M. A.; Clark, T. B. “Role of Hemilabile Diamine Ligands in the Amine-Directed C–H Borylation of Arenes” Organometallics 2015, 34, 51–55. https://pubs.acs.org/doi/10.1021/om5007837 
14. *Moore, C. M.; *Medina, C. R.; *Cannamela, P. C.; #McIntosh, M. L.; *Ferber, C. J.; Roering, A. J.; Clark, T. B. “Facile Formation of β-Hydroxyboronate Esters by a Cu-Catalyzed Diboration/Matteson Homologation Sequence” Org. Lett. 2014, 16, 6056–6059. https://pubs.acs.org/doi/10.1021/ol502767m 
13. *Guan, W.; *Michael, A. K.; *Koren-Selfridge, L.; #McIntosh, M. L.; *Scott, J. P.; Clark, T. B. “Stereoselective Formation of Trisubstituted Vinyl Boronate Esters by the Acid-Mediated Elimination of α-Hydroxyboronate Esters” J. Org. Chem. 2014, 79, 7199–7204. https://pubs.acs.org/doi/10.1021/jo500773t 
12. Crawford, K. M.; *Ramseyer, T. R.; Daley, C. J. A.; Clark, T. B. “Phosphine-Directed C–H Borylation Reactions: Facile and Selective Access to Phosphine-Substituted Arylboronate Esters” Angew. Chem., Int. Ed. 2014, 53, 7589–7593. https://doi.org/10.1002/anie.201402868 
11. Medina, C.; Carter, K. P.; Miller, M.; Clark, T. B.; O’Neil, G. W. “Stereocontrolled Synthesis of 1,3-Diols from Enones: Cooperative Lewis Base-Mediated Intramolecular Carbonyl Hydrosilylations” J. Org. Chem. 2013, 78, 9093–9101. https://pubs.acs.org/doi/10.1021/jo401293a 
10. Roering, A. J.; *Hale, L. V. A.; *Squier, P. A.; *Ringgold, M. A.; *Butler, E. R.; Clark, T. B. “Iridium-Catalyzed, Substrate-Directed C–H Borylation Reactions of Benzylic Amines” Org. Lett. 2012, 14, 3558-3561. https://pubs.acs.org/doi/10.1021/ol301635x 
9. *Query, I. P.; *Squier, P. A.; %Larson, E. M.; *Isley, N. A.; Clark, T. B. “Alkoxide-Catalyzed Reduction of Ketones with Pinacolborane” J. Org. Chem. 2011, 76, 6452–6456. 
https://pubs.acs.org/doi/10.1021/jo201142g 8. *Koren-Selfridge, L.; *Query, I. P.; *Hanson, J. A.; *Isley, N. A.; Guzei, I. A.; Clark, T. B. “Synthesis of Ruthenium Boryl Analogues of the Shvo Metal–Ligand Bifunctional Catalysts” Organometallics 2010, 29, 3896–3900. 
https://pubs.acs.org/doi/10.1021/om1005808 7. McIntosh, M. L.; *Moore, C. M.; Clark, T. B. “Copper-Catalyzed Diboration of Ketones: Facile Synthesis of α-Hydroxyboronate Esters” Org. Lett. 2010, 12, 1996–1999. 
https://pubs.acs.org/doi/10.1021/ol100468f 6. *Koren-Selfridge, L.; *Londino, H. N.; *Vellucci, J. K.; *Simmons, B. J.; Casey, C. P.; Clark, T. B. “A Boron-Substituted Analogue of the Shvo Hydrogenation Catalyst: Catalytic Hydroboration of Aldehydes, Imine, and Ketones” Organometallics 2009, 28, 2085–2090. https://pubs.acs.org/doi/10.1021/om801228m  Supervised Publications 5. Buchner, K. M.; Clark, T. B.; Loy, J. M. N.; Nguyen, T. X.; Woerpel, K. A. “Alkylidenesilacyclopropanes Derived from Allenes: Applications to the Selective Synthesis of Triols and Homoallylic Alcohols” Org. Lett. 2009, 11, 2173–2175. https://pubs.acs.org/doi/10.1021/ol900456v 
4. Casey, C. P.; Clark, T. B.; Guzei, I. A. “Intramolecular Trapping of an Intermediate in the Reduction of Imines by a Hydroxycyclopentadienyl Ruthenium Hydride: Support for a Concerted Outer Sphere Mechanism” J. Am. Chem. Soc. 2007, 129, 11821–11827. https://pubs.acs.org/doi/10.1021/ja073370x 
3. Clark, T. B.; Woerpel, K. A. “The Formation and Reactivity of Silacyclopropenes Derived from Siloxyalkynes: Stereoselective Formation of 1,2,4-Triols” Org. Lett. 2006, 8, 4109−4112. https://pubs.acs.org/doi/10.1021/ol061652g 
2. Clark, T. B.; Woerpel, K. A. “Silver-Catalyzed Silacyclopropenation of 1-Heteroatom-Substituted Alkynes and Subsequent Rearrangement Reactions” Organometallics 2005, 24, 6212−6219. https://pubs.acs.org/doi/10.1021/om050501z 
1. Clark, T. B.; Woerpel, K. A. “Formation and Reactivity of Oxasilacyclopentenes Derived from Functionalized Alkynes” J. Am. Chem. Soc. 2004, 126, 9522-9523. https://pubs.acs.org/doi/10.1021/ja047498f 
|